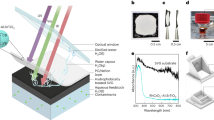
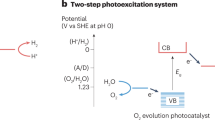
Abstract
Production of hydrogen fuel from sunlight and water, two of the most abundant natural resources on Earth, offers one of the most promising pathways for carbon neutrality1,2,3. Some solar hydrogen production approaches, for example, photoelectrochemical water splitting, often require corrosive electrolyte, limiting their performance stability and environmental sustainability1,3. Alternatively, clean hydrogen can be produced directly from sunlight and water by photocatalytic water splitting2,4,5. The solar-to-hydrogen (STH) efficiency of photocatalytic water splitting, however, has remained very low. Here we have developed a strategy to achieve a high STH efficiency of 9.2 per cent using pure water, concentrated solar light and an indium gallium nitride photocatalyst. The success of this strategy originates from the synergistic effects of promoting forward hydrogen–oxygen evolution and inhibiting the reverse hydrogen–oxygen recombination by operating at an optimal reaction temperature (about 70 degrees Celsius), which can be directly achieved by harvesting the previously wasted infrared light in sunlight. Moreover, this temperature-dependent strategy also leads to an STH efficiency of about 7 per cent from widely available tap water and sea water and an STH efficiency of 6.2 per cent in a large-scale photocatalytic water-splitting system with a natural solar light capacity of 257 watts. Our study offers a practical approach to produce hydrogen fuel efficiently from natural solar light and water, overcoming the efficiency bottleneck of solar hydrogen production.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Supporting data are available at the University of Michigan (https://doi.org/10.7302/g0xw-d923). Further details regarding the data are available from the corresponding author upon reasonable request.
References
Pinaud, B. A. et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 6, 1983–2002 (2013).
Hisatomi, T. & Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2, 387–399 (2019).
Rodriguez, C. A., Modestino, M. A., Psaltis, D. & Moser, C. Design and cost considerations for practical solar-hydrogen generators. Energy Environ. Sci. 7, 3828–3835 (2014).
Goto, Y. et al. A particulate photocatalyst water-splitting panel for large-scale solar hydrogen generation. Joule 2, 509–520 (2018).
Guan, X. et al. Efficient unassisted overall photocatalytic seawater splitting on GaN-based nanowire arrays. J. Phys. Chem. C 122, 13797–13802 (2018).
Ager, J. W., Shaner, M. R., Walczak, K. A., Sharp, I. D. & Ardo, S. Experimental demonstrations of spontaneous, solar-driven photoelectrochemical water splitting. Energy Environ. Sci. 8, 2811–2824 (2015).
Khaselev, O. & Turner, J. A. A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting. Science 280, 425–427 (1998).
Kang, D. et al. Printed assemblies of GaAs photoelectrodes with decoupled optical and reactive interfaces for unassisted solar water splitting. Nat. Energy 2, 17043 (2017).
Chowdhury, F. A., Trudeau, M. L., Guo, H. & Mi, Z. A photochemical diode artificial photosynthesis system for unassisted high efficiency overall pure water splitting. Nat. Commun. 9, 1707 (2018).
Wang, Q. et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 15, 611–615 (2016).
Maeda, K. et al. Noble-metal/Cr2O3 core/shell nanoparticles as a cocatalyst for photocatalytic overall water Splitting. Angew. Chem. Int. Edn 45, 7806–7809 (2006).
Takata, T. et al. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 581, 411–414 (2020).
Fountaine, K. T., Lewerenz, H. J. & Atwater, H. A. Efficiency limits for photoelectrochemical water-splitting. Nat. Commun. 7, 13706 (2016).
Kibria, M. G. et al. Visible light-driven efficient overall water splitting using p-type metal-nitride nanowire arrays. Nat. Commun. 6, 6797 (2015).
Licht, S. Solar water splitting to generate hydrogen fuel: photothermal electrochemical analysis. J. Phys. Chem. B 107, 4253–4260 (2003).
Moses, P. G. & Walle, C. G. V. D. Band bowing and band alignment in InGaN alloys. Appl. Phys. Lett. 96, 021908 (2010).
Wang, Y., Wu, Y., Sun, K. & Mi, Z. A quadruple-band metal–nitride nanowire artificial photosynthesis system for high efficiency photocatalytic overall solar water splitting. Mater. Horiz. 6, 1454–1462 (2019).
Kibria, M. G. et al. Tuning the surface Fermi level on p-type gallium nitride nanowires for efficient overall water splitting. Nat. Commun. 5, 3825 (2014).
Guan, X. et al. Making of an industry-friendly artificial photosynthesis device. ACS Energy Lett. 3, 2230–2231 (2018).
Xu, Y. & Schoonen, M. A. A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 85, 543–556 (2000).
Zhou, P., Yu, J. & Jaroniec, M. All-solid-state Z-scheme photocatalytic systems. Adv. Mater. 26, 4920–4935 (2014).
Nishiyama, H. et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 598, 304–307 (2021).
Zhang, B., Daniel, Q., Cheng, M., Fan, L. & Sun, L. Temperature dependence of electrocatalytic water oxidation: a triple device model with a photothermal collector and photovoltaic cell coupled to an electrolyzer. Faraday Discuss. 198, 169–179 (2017).
Jin, B. et al. Promoting oxygen evolution reaction of Co-based catalysts (Co3O4, CoS, CoP, and CoN) through photothermal effect. Small 15, 1903847 (2019).
Tembhurne, S., Nandjou, F. & Haussener, S. A thermally synergistic photo-electrochemical hydrogen generator operating under concentrated solar irradiation. Nat. Energy 4, 399–407 (2019).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Zhang, Y. et al. Rate-limiting O–O bond formation pathways for water oxidation on hematite photoanode. J. Am. Chem. Soc. 140, 3264–3269 (2018).
Hisatomi, T., Takanabe, K. & Domen, K. Photocatalytic water-splitting reaction from catalytic and kinetic perspectives. Catal. Lett. 145, 95–108 (2015).
Wang, M., Zhen, W., Tian, B., Ma, J. & Lu, G. The inhibition of hydrogen and oxygen recombination reaction by halogen atoms on over-all water splitting over Pt–TiO2 photocatalyst. Appl. Catal. B 236, 240–252 (2018).
Wang, M., Li, Z., Wu, Y., Ma, J. & Lu, G. Inhibition of hydrogen and oxygen reverse recombination reaction over Pt/TiO2 by F− ions and its impact on the photocatalytic hydrogen formation. J. Catal. 353, 162–170 (2017).
Zhang, Y., Hu, H., Huang, X. & Bi, Y. Photo-controlled bond changes on Pt/TiO2 for promoting overall water splitting and restraining hydrogen–oxygen recombination. J. Mater. Chem. A 7, 5938–5942 (2019).
Verhallen, P. T. H. M., Oomen, L. J. P., Elsen, A. J. J. M. V. D., Kruger, J. & Fortuin, J. M. H. The diffusion coefficients of helium, hydrogen, oxygen and nitrogen in water determined from the permeability of a stagnant liquid layer in the quasi-steady state. Chem. Eng. Sci. 39, 1535–1541 (1984).
Nguyen, H. P. T., Djavid, M., Cui, K. & Mi, Z. Temperature-dependent nonradiative recombination processes in GaN-based nanowire white-light-emitting diodes on silicon. Nanotechnology 23, 194012 (2012).
Kibria, M. G. et al. One-step overall water splitting under visible light using multiband InGaN/GaN nanowire heterostructures. ACS Nano 7, 7886–7893 (2013).
Kibria, M. G. & Mi, Z. Artificial photosynthesis using metal/nonmetal-nitride semiconductors: current status, prospects, and challenges. J. Mater. Chem. A 4, 2801–2820 (2016).
Benton, J., Bai, J. & Wang, T. Utilisation of GaN and InGaN/GaN with nanoporous structures for water splitting. Appl. Phys. Lett. 105, 223902 (2014).
Aryal, K., Pantha, B. N., Li, J., Lin, J. Y. & Jiang, H. X. Hydrogen generation by solar water splitting using p-InGaN photoelectrochemical cells. Appl. Phys. Lett. 96, 052110 (2010).
Fabian, D. M. et al. Particle suspension reactors and materials for solar-driven water splitting. Energy Environ. Sci. 8, 2825–2850 (2015).
Hisatomi, T., Kubota, J. & Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 43, 7520–7535 (2014).
Pelá, R. R. et al. Accurate band gaps of AlGaN, InGaN, and AlInN alloys calculations based on LDA-1/2 approach. Appl. Phys. Lett. 98, 151907 (2011).
Vurgaftman, I., Meyer, J. R. & Ram-Mohan, L. R. Band parameters for III–V compound semiconductors and their alloys. J. Appl. Phys. 89, 5815–5875 (2001).
Kuykendall, T., Ulrich, P., Aloni, S. & Yang, P. Complete composition tunability of InGaN nanowires using a combinatorial approach. Nat. Mater. 6, 951–956 (2007).
Young, J. L. et al. Direct solar-to-hydrogen conversion via inverted metamorphic multi-junction semiconductor architectures. Nat. Energy 2, 17028 (2017).
Cheng, W.-H. et al. Monolithic photoelectrochemical device for direct water splitting with 19% efficiency. ACS Energy Lett. 3, 1795–1800 (2018).
Li, X. et al. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 3, 2485–2534 (2015).
Zou, Z., Ye, J., Sayama, K. & Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 414, 625–627 (2001).
Diffey, B. Solar spectral irradiance and summary outputs using excel. Photochem. Photobiol. 91, 553–557 (2015).
Shaner, M. R., Atwater, H. A., Lewis, N. S. & McFarland, E. W. A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ. Sci. 9, 2354–2371 (2016).
Kim, J. H., Hansora, D., Sharma, P., Jang, J.-W. & Lee, J. S. Toward practical solar hydrogen production – an artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 48, 1908–1971 (2019).
Lewis, N. S. Research opportunities to advance solar energy utilization. Science 351, aad1920 (2016).
Tachibana, Y., Vayssieres, L. & Durrant, J. R. Artificial photosynthesis for solar water-splitting. Nat. Photon. 6, 511–518 (2012).
Liao, L. B. et al. Efficient solar water-splitting using a nanocrystalline CoO photocatalyst. Nat. Nanotechnol. 9, 69–73 (2014).
Kim, J. H. et al. Wireless solar water splitting device with robust cobalt-catalyzed, dual-doped BiVO4 photoanode and perovskite solar cell in tandem: a dual absorber artificial leaf. ACS Nano 9, 11820–11829 (2015).
Liu, W. et al. Single-site active cobalt-based photocatalyst with a long carrier lifetime for spontaneous overall water splitting. Angew. Chem. Int. Edn 56, 9312–9317 (2017).
Liu, C., Tang, J., Chen, H. M., Liu, B. & Yang, P. A fully integrated nanosystem of semiconductor nanowires for direct solar water splitting. Nano Lett. 13, 2989–2992 (2013).
Wang, Q. et al. Oxysulfide photocatalyst for visible-light-driven overall water splitting. Nat. Mater. 18, 827–832 (2019).
Acknowledgements
This work was supported by National Science Foundation under grant CBET 1804458, the Department of Defense (contracts W56HZV-20-C-0025 and W56HZV-21-C-0076), the Michigan Translational Research and Commercialization (MTRAC) Innovation Hub, and the Blue Sky Program in the College of Engineering at the University of Michigan, and by Army Research Office Award W911NF2110337. Y.M. was a visiting student from Central South University and acknowledges financial support by China Scholarship Council in 2019-2021. We also thank Q. Gan for drawing Extended Data Fig. 4e.
Author information
Authors and Affiliations
Contributions
P.Z. designed the photocatalyst and performed the photocatalytic reactions. I.A.N. synthesized the InGaN/GaN NWs. Y.X., K.S. and P.W. performed SEM and TEM. P.Z., Y.M., B.Z. and Z.Y. performed the outdoor photocatalytic experiments. Z.M. supervised the whole project. P.Z. and Z.M. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
Some intellectual properties related to this work have been licensed to NS Nanotech, Inc. and NX Fuels, Inc., which were co-founded by Z.M. The University of Michigan and Mi have a financial interest in both companies.
Peer review
Peer review information
Nature thanks Jennifer Strunk, Kazuhiro Takanabe, Martijn Zwijnenburg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Bandgap determination.
SEM-CL images of InGaN/GaN nanowires. The high-energy electrons from the scanning electron microscope were used to photo-excite photocatalyst to produce photogenerated electrons and holes. The recombination of photogenerated electrons and holes leads to the cathodoluminescence phenomenon, which could be used to estimate the bandgap of the photocatalyst. A bandwidth of 5 nm was used to capture these monochromatic CL images. The luminescence bands at 445–545 nm revealed the visible-light response of InGaN/GaN nanowires.
Extended Data Fig. 2 Light-response characterization and theoretical maximum STH.
a, UV-vis diffuse reflectance spectroscopy of InGaN/GaN suggests that the visible-light-response range of InGaN/GaN nanostructures is extended to 632 nm (1.96 eV). b, Band diagram of InGaN/GaN segments. c, d, Wavelength-dependent theoretical maximum STH in (c) natural solar light and (d) simulated solar light produced by a Xe lamp equipped with a standard AM1.5G filter from Newport Corporation. The red dashed line corresponds to a photocatalyst with bandgap of 1.96 eV (632 nm).
Extended Data Fig. 3 Temperature-controllable photocatalytic OWS system and reaction condition optimization.
a, Temperature-controllable photocatalytic OWS system. b, Schematic illustration of the temperature-controllable photocatalytic OWS system. A double-layer chamber was used to perform the temperature-controllable photocatalytic OWS. The circulating water provided by a PolyScience 7L heated circulator was used to control the temperature of the reaction chamber. c, STH of InGaN/GaN NWs with different Rh/Cr2O3/Co3O4 precursor volumes at 70 °C. x (x = 2, 3, 4, 5, 6) μl of 0.2 mol l−1 Na3RhCl6, x μl of 0.2 mol l−1 K2CrO4, x μl of 0.2 mol l−1 Co(NO3)2·6H2O were used in the photodeposition of cocatalyst. The photodeposition method was shown in the experimental section. The optimized contents (µg cm−2) of cocatalyst obtained by ICP was shown in the Extended Data Table 1. d, STH of Rh/Cr2O3/Co3O4-InGaN/GaN NWs under different light intensity at 70 °C. 1 sun: 100 mW cm−2. The STH of Rh/Cr2O3/Co3O4-InGaN/GaN NWs first increased with cocatalyst content and then reached a maximum value. A further increase on the cocatalyst content could not improve the STH. The optimized cocatalyst content was utilized in all subsequent experiments. The light intensity on the photocatalyst wafer was adjusted from 1,000 mW cm−2 to 3,800 mW cm−2 at 70 °C. The results showed that the STH was not observably changed with the light intensity larger than 13 suns at the same temperature (70 °C). Error bars indicate standard deviation for three measurements.
Extended Data Fig. 4 Self-heated photocatalytic OWS system.
a, Self-heated photocatalytic OWS system. b, Schematic parameters of the self-heated photocatalytic OWS system. c, d, Thermodynamic parameters of the reaction system (c) without and (d) with a heat-insulating layer. The heat-insulating layer (thickness of approximately 0.5 cm) consisted of ordinary A4 printing paper. The wall thickness of the Prexy chamber was approximately 0.3 cm. e, Synergetic effect mechanism of promoting forward hydrogen–oxygen evolution and inhibiting the reverse hydrogen–oxygen recombination in the photocatalytic OWS. The UV-vis light was responsible for the production of photogenerated electrons and holes via the photoexcitation of InGaN/GaN semiconductor, which can further cause the redox of water. Although the infrared light was non-effective for the photoexcitation of InGaN/GaN, it could produce a substantial thermal effect to promote hydrogen/oxygen production and simultaneously inhibit the hydrogen–oxygen recombination. In this mechanism, the infrared light indirectly, but substantially improved the utilization efficiency of UV-vis light by enhancing the surface catalytic hydrogen/oxygen production, which finally contributed to the maximizing of STH. A thermal transfer balance existed in our system during photocatalytic reaction, which was influenced by the thermal conductivity coefficients of materials used in the reaction system. The role of the heat-insulating layer was to produce a larger temperature difference between ambient and internal environment of reaction system. The thermal conductivity coefficients of Prexy glass, heat-insulating layer and water were 1,143, 50 and 600 mW m−1 K−1, respectively. In the absence of the heat-insulating layer, the temperature of water in the chamber was maintained at roughly 50 °C. However, with the addition of low thermal-conductivity heat-insulating layer, the temperature of reaction system could be increased to approximately 70 °C under the same IR input (428 mW). The species and content of impurities in tap water can be found on the website of Ann Arbor Water Treatment Services Unit (https://www.a2gov.org/departments/water-treatment/Documents/water_quality_report_2020.pdf).
Extended Data Fig. 5 Stability evaluation.
a, Time-course photocatalytic production of H2 and O2 in deionized water on Rh/Cr2O3/Co3O4-InGaN/GaN NWs. b, Activity test after 74-hour photocatalytic OWS reaction. c–e, FESEM (c), HAADF-STEM (d) and elemental mapping (e) of Rh/Cr2O3/Co3O4-InGaN/GaN NWs after 80-hour photocatalytic OWS reaction.
Extended Data Fig. 6 Mechanism investigation of temperature-dependent photocatalytic OWS system.
a, Photocatalytic OWS system without circulating water layer or heat-insulating layer. b, Corresponding time-course production of stoichiometric H2 and O2 in deionized water on Rh/Cr2O3/Co3O4-InGaN/GaN NWs. c, Photocatalytic hydrogen production with methanol (20vol%) as electron donor at 70 °C. d, Photocatalytic oxygen production with KIO3 (0.4 mol l−1) as electron acceptor at 70 °C. e, Time-course production of stoichiometric H2 and O2 in deionized water on Rh/Cr2O3/Co3O4-InGaN/GaN NWs at 70 °C in 360 min. In the photocatalytic overall water splitting, the hydrogen–oxygen production (2H2O → 2H2 + O2) often competes with hydrogen–oxygen recombination (2H2O ← 2H2 + O2). Commonly, the rate of chemical reaction strongly depends on the concentration of reactant. Thus, in the initial stage of photocatalytic water splitting (2H2O ↔ 2H2 + O2), the reaction rate of hydrogen–oxygen production was theoretically higher than that of hydrogen–oxygen recombination. However, with increasing hydrogen/oxygen concentration, the hydrogen–oxygen production and recombination would reach a balance. As a result, the amount of produced hydrogen and oxygen could not be further increased with reaction time. Especially, for the continuous flow-type water splitting system, the produced hydrogen and oxygen would be full of chamber under atmospheric pressure before transferred. This easily leads to a high-concentration hydrogen and oxygen in the chamber, which unavoidably leads to the more severe hydrogen–oxygen recombination. f, Time-course production of H2 and O2 in deionized water on Rh/Cr2O3/Co3O4-InGaN/GaN NWs with and without light source at 70 °C. Light source: 3,800 mW cm−2 produced by a 300 W Xe lamp equipped with AM1.5G filter. Sample: 0.8 cm × 0.8 cm Rh/Cr2O3/Co3O4-loaded InGaN/GaN nanowires wafer. Error bars indicate standard deviation for three measurements.
Extended Data Fig. 7 Theoretical simulation.
a–c, Geometry structures of reaction steps in hydrogen–oxygen recombination on the cocatalyst (a) Co3O4, (b) Rh and (c) Cr2O3. The results suggested that the desorption of product water was the rate-determining step in hydrogen–oxygen recombination on Co3O4 and Cr2O3. Besides, this also implied that water tended to be adsorbed on Co3O4 and Cr2O3, which inhibited the further hydrogen–oxygen adsorption or recombination on them.
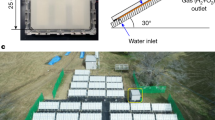
Extended Data Fig. 8 Photocatalytic OWS under natural concentrated solar light.
a, Schematic parameter illustration of outdoor photocatalytic OWS system. b, Reaction temperature and natural light intensity in the outdoor test. The thickness of heat-insulating layer (ordinary A4 printing paper) and wall of chamber were approximately 0.5 cm and approximately 0.3 cm, respectively. The outdoor photocatalytic OWS was conducted from 5 November 2020 to 7 November 2020. The average natural solar light intensity was determined to be approximately 85 mW cm−2 between 11 pm and 3 pm by a thermopile detector (919P, Newport) with a 5 cm2 detector. c, Activity test after 140-min outdoor photocatalytic OWS reaction. Each cycle: 10 min.
Supplementary information
Supplementary Video 1
The dynamic process of gas (hydrogen, oxygen and vapour) production in direct view. Conditions: 4 cm × 4 cm Rh/Cr2O3/Co3O4-loaded InGaN/GaN nanowires under concentrated natural solar light (about 16,070 mW cm−2).
Supplementary Video 2
The dynamic process of gas (hydrogen, oxygen and vapour) production in filter view (green). Conditions: 4 cm × 4 cm Rh/Cr2O3/Co3O4-loaded InGaN/GaN nanowires under concentrated natural solar light (about 16,070 mW cm−2).
Supplementary Video 3
The dynamic process of water vapour production in filter view. Conditions: 4 cm × 4 cm silicon wafer under concentrated natural solar light (about 16,070 mW cm−2).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, P., Navid, I.A., Ma, Y. et al. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 613, 66–70 (2023). https://doi.org/10.1038/s41586-022-05399-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05399-1
This article is cited by
-
Large electronegativity differences between adjacent atomic sites activate and stabilize ZnIn2S4 for efficient photocatalytic overall water splitting
Nature Communications (2024)
-
Electrolyte-assisted polarization leading to enhanced charge separation and solar-to-hydrogen conversion efficiency of seawater splitting
Nature Catalysis (2024)
-
A soft-contact hybrid electromagnetic–triboelectric nanogenerator for self-powered water splitting towards hydrogen production
Nano Research (2024)
-
Approaching the commercial threshold of solar water splitting toward hydrogen by III-nitrides nanowires
Frontiers in Energy (2024)
-
Nanoparticle Exsolution on Perovskite Oxides: Insights into Mechanism, Characteristics and Novel Strategies
Nano-Micro Letters (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.