The Inflation Reduction Act
and Medicare Drug Price “Negotiation”


Overview
Signed into law in 2022, the Inflation Reduction Act (IRA) put in place policies that give the government unchecked authority to set prices for certain medicines covered by Medicare. These policies are expected to have a negative impact on access to medicines covered by Medicare Part B and Part D, in addition to discouraging drug development.
While the law contains some important policies starting this year that lower patient out-of-pocket costs in Medicare Part D, including a $2,000 annual out-of-pocket cap and allowing seniors to spread their costs throughout the year, it includes harmful provisions that must be urgently addressed to ensure lasting patient access to innovative, safe and effective treatments.
The IRA’s Harmful Consequences A penalty on pills is not what seniors were promised.
The IRA includes a “pill penalty” which unfairly discriminates against small molecule medicines — medicines that typically come in pill form, like a capsule or tablet — by allowing them to be selected for government price setting years before other medicines. By doing so, the law disincentivizes researchers from developing these medicines despite the immense value they provide to patients.
We are already seeing the impact:
Early-stage funding for small molecule development has fallen nearly 70% since the IRA was introduced.
Today, millions of patients count on these medicines to manage their condition and recover from illness. Here’s what patients stand to lose if the IRA’s harmful pill penalty continues to stifle innovation:

Affordability
Pills eliminate the need for additional expenses related to physician administration and hospitals that can be associated with specialized administration of other more complex therapies. Once generic versions become available, they can be purchased at a fraction of the original price, with an average copay of just $6.16 and generic competition driving prices down by nearly 85%.
The IRA will jeopardize critical post-approval research.
Innovation doesn’t end once a medicine is first approved by the FDA. After initial approval, researchers often pursue new uses of approved medicines to address patient needs, often to treat different diseases and patient populations, as well as new dosage forms and formulations which can improve delivery methods or help patients better manage their conditions.
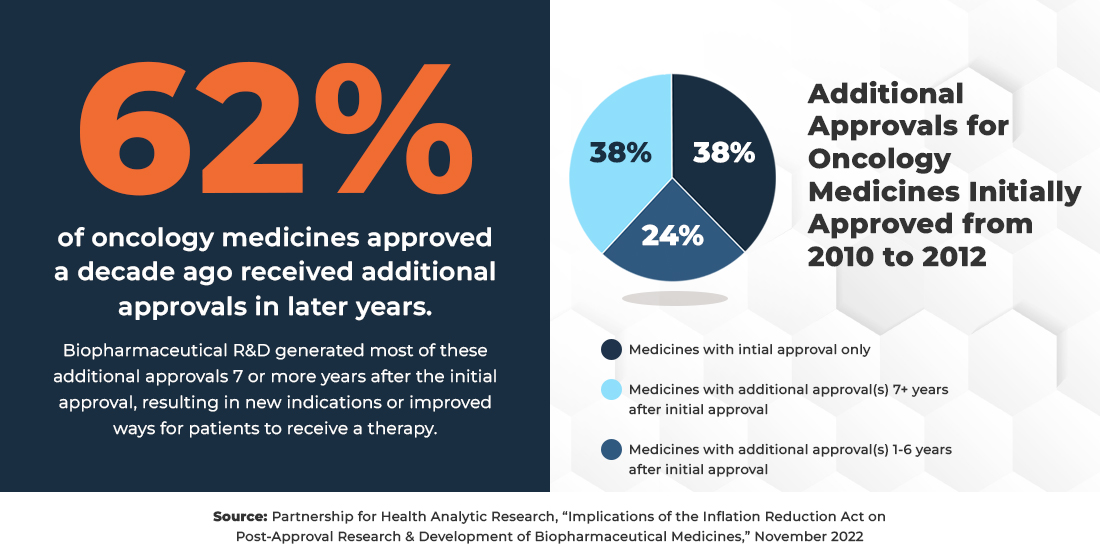
Because the price setting provisions in the IRA can begin as early as seven years after a medicine is initially approved, they ignore the critical R&D that continues in the years following a medicine’s approval. Unfortunately, the law’s price-setting pressures mean that companies may face the difficult decision to not explore new uses for approved medicines as well as new dosage forms and formulations as they may be too costly to pursue.
Post-approval R&D delivers for patients.

More barriers to medicines and higher costs for patients.
What Medicare Part D patients pay depends on many factors, including prescription drug benefit design. Insurers and pharmacy benefit managers (PBMs) have substantial control over what that benefit design looks like, what hoops people must jump through, and what patients pay at the pharmacy counter. The IRA did nothing to rein in insurer and PBM tactics that harm patients. Not only do patients not benefit from rebates and discounts at the pharmacy counter, but their cost-sharing for medicines can sometimes exceed their plans’ net costs. As a result of changes to the Part D structure, insurers are likely to implement stricter utilization management and move more medications to higher-cost, non-preferred, and specialty tiers in their formularies. The result is more barriers to medicines and higher out of pocket costs for beneficiaries enrolled in Part D.
- 60% of insurers say they expect to impose more utilization managements as a result of price setting.
- 78% of insurers say will limit available therapeutic options in Part D.
- Some beneficiaries taking price-set drugs are facing monthly cost increases up to 76% for drugs impacted by the pill penalty.
Patient Perspective
Higher costs and more barriers weren’t what patients were promised.
Related Resources

Blog How the IRA is impacting the generic drug market
April 8, 2025
